As a the official journal of the Association for Research in Otolaryngology (ARO), JARO publishes peer-reviewed research findings focused on the auditory and vestibular systems. JARO reflects the diversity of research presented at the ARO Mid-Winter Meeting and welcomes submissions describing original experimental research that investigates the mechanisms underlying problems of basic or clinical significance. Example research areas include: molecular biology, genetics related to hearing and balance mechanisms, biochemistry, cochlear anatomy and physiology, opto- and chemo-genetics, vestibular anatomy and physiology, electrophysiology, middle ear anatomy and modeling, mechanotransduction, computational central anatomy, behavior and physiology, psychoacoustics in normal and hearing-impaired subjects, and studies of hearing perception in cochlear implant patients.
Why publish with us?
- As the official journal of Association for Research in Otolaryngology, we are committed to ensuring high visibility for your publication through the society membership, libraries and social media.
- Our dedicated and expert editorial teams will support you through the peer review process.
- We do not apply submissions fees or page charges. Through Springer Compact agreements, authors from participating institutions can publish Open Choice at no cost to the authors.
Types of articles
JARO considers the following article types:
Note: For all articles (except editorials, commentaries, reviews, and symposia), no limits are imposed on article length, on the number of figures and tables, with unlimited references. We however encourage authors to maintain the message to the essential. While the main article should contain key information, supportive information and evidence should be provided as supplemental figures, tables or data.
- Original Research Article Please go to organization and content of papers section below for information on structuring your article.
- Systematic Reviews Authors must register their study in a publicly accessible database (e.g., PROSPERO, Open Science Framework, Research Registry), and include the registration number in the manuscript. JARO will refuse to consider systematic reviews that have been registered after data extraction has begun. The study protocol should be submitted as supplementary material, or its reference included in the methods. There is no need to contact the Editor-in-Chief before submitting a systematic review; please upload the manuscript in the usual way, along with a PRISMA or MOOSE checklist.
- Meta-analyses A maximum of 4000 words in the main text with unlimited references. We recommend that authors register their study in a publicly accessible database and submit the study protocol as supplementary material. There is no need to contact the Editor-in-Chief before submitting a meta-analysis; please upload at https://mc.manuscriptcentral.com/JARO in the usual way.
For meta-analyses of randomized controlled trials, follow the PRISMA reporting guidelines—include a flow diagram in your manuscript and submit a completed PRISMA checklist. For meta-analyses of observational studies in epidemiology, follow the MOOSE reporting guidelines and submit a completed MOOSE checklist.
- Randomized Controlled Trials In accordance with guidelines issued by the ICMJE, JARO requires registration of clinical trials in a public trials registry at or before the time of first patient enrolment. A clinical trial is defined as any research project that prospectively assigns people to an intervention, with or without concurrent comparison or control groups, to study the cause-and-effect relationship between a health-related intervention and a health outcome. Health-related interventions are those used to modify a biomedical or health-related outcome; examples include drugs, surgical procedures, devices, behavioral treatments, dietary interventions, quality improvement interventions and process-of-care changes. Health outcomes are any biomedical or health-related measures obtained in patients or participants, including pharmacokinetic measures and adverse events.
Purely observational studies (those in which the assignment of the medical intervention is not at the discretion of the investigator) do not require registration.
The ICMJE accepts registration in any registry that is a primary register of the WHO International Clinical Trials Registry Platform or in ClinicalTrials.gov. The trial registry number should be included at the end of the Abstract.
Reports of randomized controlled trials should include the checklist items set out in the CONSORT guidelines, as well as a patient flow diagram. See the CONSORT website for further details. Authors must submit a completed CONSORT 2010 checklist, along with the original trial protocol (including statistical analyses to be undertaken). For reports of non-pharmacological treatment interventions, please use the appropriate extension of the CONSORT statement.
For secondary analyses of randomized controlled trials or observational studies, please complete either a CONSORT or a STROBE checklist, as appropriate. Reference can be made in the checklist and the current paper to previous publications that describe the study in more detail. Any sections of the checklist that do not apply to the current study can be marked ‘not applicable’ (NA). Please note that a STROBE checklist might be more suitable where a cohort from a previous randomized controlled trial is used to answer a different research question.
- Genetic Association Studies There is a widely accepted need to improve the robustness of published genetic association findings. We also need to provide the readership of the journal with information that allows a more complete assessment of the biological significance of the findings reported in these kinds of manuscripts. Submissions to JARO should, therefore, pay careful attention to the following fundamental issues of study design. It is not intended that these represent absolute criteria for publication in JARO (we do not want to block otherwise interesting studies that fail to meet one or another of these). However, these guidelines set out the main factors that we expect our reviewers and Associate Editors to use in evaluating the quality of the manuscripts we receive. Submissions to JARO should, therefore, pay careful attention to the following fundamental issues of study design: size, multiple testing, functional data, whole gene studies, replication, phenotypes and other technical requirements.
Size Studies should include sufficient samples to have power to detect effect sizes that are reasonable given current understanding of the genetic architecture of complex traits. Power calculations should be included that make explicit the effect sizes that the study was powered to detect; such power calculations should guide the interpretation of the data. Wherever possible, all available samples should be typed: results based on only a portion of a larger sample are of limited interest.
Multiple testing Genetic association studies often involve testing of a large number of hypotheses (e.g., multiple single nucleotide polymorphisms [SNPs] or haplotypes; multiple phenotypes; multiple analytical models; testing of multiple strata such as male/female, lean/obese). Manuscripts should feature explicit discussion of the consequences of multiple hypothesis testing for the interpretation of the findings. Assessments of the significance of the findings should be related to the study-wide (or genome-wide) significance.
Functional data Functional data (e.g., demonstration that a SNP alters expression) can strengthen association findings, but the functional assays must have demonstrable relevance to the phenotype showing the association. Good functional data do not compensate for a poor association study.
Whole gene studies Single SNP studies are acceptable where the SNPs typed have strong prior claims for involvement in the trait of interest. However, where feasible, studies should attempt to examine genome sequence variation across a gene.
Replication Replication is highly desirable for all association studies, particularly for studies where extensive multiple testing means that study-wide significance is not clear. However, replication should only be claimed when it addresses the same variant, phenotype and genetic model (all too often other phenotypes or variants within a gene are offered as evidence of replication).
Phenotypes Authors should explicitly justify why the samples typed are well-suited to address the particular hypothesis posed. Care needs to be taken in the definition of cases using standardized criteria, and in the selection of appropriate control samples.
Positive/negative studies Well-performed association studies that represent “significant negative” findings are welcome provided the gene examined has clear relevance to disease pathogenesis (or has been implicated on the basis of prior association data).
Technical requirements The information provided by a manuscript can be improved if certain technical requirements are observed. Where relevant, we ask that authors:
-
-
- Provide rs numbers for all variants reported (these are quite easy to obtain for novel variants). Where these are provided, details of the assay (primer sequences, PCR conditions) can be kept brief;
- Provide explicit details of the measures taken to ensure genotyping accuracy (including, for example, % successful genotype calls, number of duplicated genotypes, % correspondence);
- Provide approved HUGO gene names in the appropriate case and italics;
- Use standard terminology for variants (see http://www.hgvs.org/mutnomen/);
- Describe LD relationships between typed variants;
- Provide information on departures from Hardy–Weinberg equilibrium (HWE), not only as a check for possible genotyping errors, but also because methods assuming HWE may be employed in the downstream association analyses (e.g., haplotype inference using the EM algorithm/single-point analyses testing the multiplicative model);
- Provide raw genotype frequencies (i.e., allele frequencies alone are not sufficient);
- Provide the criteria they have used to select tagSNPs. Authors should also carry out association analyses consistent with the tagging method employed, e.g., if an aggressive multimaker tagging approach has been followed, appropriate analyses are required to retrieve all the information captured;
- Denote the boundaries they have considered when studying a gene of interest (e.g., 5 kb upstream of transcription initiation, etc.) and indicate which portions of the gene have been examined (e.g. exons and exon / intron boundaries).
-
- Database Studies For studies that involve the use of patient data from databases, or routinely collected health data, authors should complete either a STROBE checklist or a RECORD checklist. Where data are collected for a more-or-less specific research purpose, STROBE is the most appropriate, whereas RECORD is more suitable for routinely collected data.
- Technical Advances There are reports on new methods in auditory and vestibular research. They are meant to highlight new approaches to experimental studies that are specific to the field, including new analysis methods. Evidence that the advances can lead to new insights is a consideration in the review. “How I do it” descriptions, or reviews of established techniques, are unlikely to be considered. Reports may be in a review-like format or a research article format. A pre-submission inquiry to the Editor-in-Chief is recommended before submitting a Technical Advances manuscript to confirm that the work is likely to be considered.
- Editorials; Commentaries; Reviews; Symposia: By invitation only, or after inquiry with the Editor-in-Chief.
- Editorials Editorials are invited papers at the discretion of the Editor-in-Chief. They should not exceed 1,500 words and 10 references. No abstract or keywords are required.
- Commentaries Commentaries are invited short papers referring to articles published in the journal or other journals. They should not exceed 3,000 words. An unstructured single-paragraph abstract of not more than 50 words is required, and keywords are mandatory. The text should not be divided into separate sections. The title of the Commentary should be related to the article under discussion. Please do not include the following text in the title of the Commentary: “Commentary to….” The article to which the Commentary refers must be formally quoted in the text and appropriately cited in the list of the references. If the Commentary addresses an original article published in the journal, the authors of the original articles will have the opportunity to reply to the Commentary concerning their article. JARO will endeavor to publish the reply alongside the comment. The title of the replying letter should be: "TITLE OF THE ORIGINAL ARTICLE: reply".
- Review Article Review articles are invited papers by recognized authorities on particular topics within the Aims and Scope of the journal. An unstructured single-paragraph Abstract of not more than 250 words is required, and keywords are mandatory. Review articles should not exceed 10,000 words.
- Symposia/Proceedings These papers are short (2500–4000 words), multi-author reviews of symposia or workshops held at various meetings, such as the Midwinter meeting of the Association for Research in Otolaryngology. An unstructured single-paragraph Abstract of not more than 250 words is required, and keywords are mandatory. It may have figures and references. Topics should fit within the Aims and Scope of the journal.
- Clinical case studies, pharmaceutical screens and methods papers are not encouraged unless they include significant new findings as well.
Title page
Arrange as follows:
Article type Original article, Review, …
Title Short and informative—no abbreviations, 12 words maximum.
Authors All authors must fulfill the following criteria defined by the ICMJE as Uniform Requirements for Manuscripts Submitted to Biomedical Journals: (1) substantial contributions to conception and design, acquisition of data or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content; (3) final approval of the version to be published; and (4) agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Include full first name, initial(s) of middle name(s) and full surname; superscripts after names refer to affiliations; authors with the same affiliation are given the same superscript. Example: Zaria R. Kim1, Soren T. Smith2 and Maya Tonelli1 .
Authors whose names are normally composed of non-Latin characters can now include their names in parentheses after a transliterated version, for example, Jingbing Xue (薛晶冰). These non-Latin characters must be represented in Unicode characters. Unicode will be allowed only for the original form of a transliterated name, and no additional information (such as degrees) should be included in this format.
Affiliations Institute, town, country in accordance with the superscripts after the authors’ names. Do not include street names, post office boxes or postal codes. Include ORCID identifiers if available.
A short title for the running head of the article (< 50 characters including spaces);
Correspondence address Complete contact information of the corresponding author, including telephone number, fax number and e-mail address. If the corresponding author will be unavailable for a significant period, also specify an alternate author and provide the same information.
Tweet We are committed to making your research as widely accessible as possible and will tweet (@JARO_News) about accepted articles (reviews, original articles and short communications). Please include a tweet along with institutional and personal twitter handles and relevant hashtags (maximum of 250 characters, including spaces) on the title page of your article. Your tweet can be based on the title of your paper or the main finding(s). We will include your graphical abstract with the tweet.
Abstract and Keywords (on the second page)
Abstracts () should be structured into four paragraphs as follows: (1) Aims/hypothesis, (2) Methods, (3) Results and (4) Conclusions/interpretation. The Abstract should contain data to support the main results of your paper. Please do not include unexplained abbreviations, nor references. There is no upper word limit.
Please include numerical data in your abstract to support the main findings of your paper, if appropriate. (Our word limit for Abstracts is flexible.) Please make sure the data in your Abstract can also be found easily in the Results section of your paper or in the tables, and make sure that data are reported consistently in the Abstract and Results, and to the same number of decimal places.
For clinical trials, the trial registry number should be included at the end of the Abstract.
For randomized controlled trials, Abstracts should include the checklist items set out in the CONSORT guidelines.
Acronyms
Define acronyms at first mention in the Abstract and text.
Body of the Text (on the third page)
Organize the body of the text into: Introduction, Materials and Methods, Results, and Discussion. JARO does not have a page limit or word limits. Insert continuous line numbers from the first page.
Each figure should be uploaded individually as a separate file. Tables are included before the legends. All supplemental information (aside from very large tables of data or Excel files) should be compiled into a PDF and uploaded as a single file (e.g., Supplemental Figures, Supplemental Tables, Supplemental Data).
Introduction
Introductions are less than 800 words. Provide relevant and succinct background on the study and explain its purpose and importance. It should include neither results nor conclusions.
Materials and Methods
- Methods include all procedures and instructions, manipulations of subject material, selection of subjects, methods used to compute stimuli, versions of programs used, specific hardware, etc. Materials include samples, animal sources and lineage, special storage of key reagents, identification of antibodies by lot, complete primer sequences, etc. The goal of the Materials and Methods section is to allow studies to be replicated in a lab that is similarly equipped.
- References to prior methods papers may be made, but only if the original method has been followed exactly, and is completely and clearly described in the prior material. Otherwise, JARO requires that the methods be rewritten (to avoid plagiarism) and be described completely. The purpose of complete methods is to aid in reproducibility and to allow readers to fully understand how the study was done.
- In human studies, detailed descriptions should be provided of the individuals’ clinical characteristics (including the type of hearing loss, e.g., sensorineural hearing loss, conductive, congenital) upon which individuals were classified. Relative body weight should be expressed in terms of body mass index, i.e., (weight in kg)/(height in meters)2 (not % ideal body weight).
- Informed consent and ethics committee approval A paper describing experimental work in humans must (1) indicate that informed consent has been obtained from patients where appropriate and (2) include a statement that the responsible ethics committee (institutional review board) has given approval, and/or indicate that the reported investigations have been carried out in accordance with the principles of the Declaration of Helsinki as revised in 2008. For studies in children/minors, where informed consent has been provided by the parent or guardian, assent should also be given directly by the child, where practical. Please mention in your paper whether this has been sought and obtained. Do not use participant names, initials or hospital numbers, especially in illustrative material. For studies involving human embryos, gametes and stem cells, please see Springer’s policy guidelines and ensure that the manuscript includes an ethics statement identifying the institutional and/or national research ethics committee approving the experiments and describing any relevant details. Authors should confirm that informed consent was obtained from all recipients and/or donors of cells or tissues, where necessary, and describe the conditions of donation of materials for research, such as human embryos or gametes. Uniform requirements should be followed for ethical standards.
- In animal studies, describe animal experiments in detail according to the full ARRIVE guidelines and submit a completed version of the ARRIVE author checklist with your manuscript. Indicate the species and, where appropriate, the strain of the animal; the total number of animals used throughout the study; the number of animals per experimental group; the experimental design including statistical design and analysis; randomization and blinding methods; other pertinent details relating to the lifetime experience of the animals, including housing and care; refinements of experimental procedures to reduce suffering; pain management; humane endpoints; and euthanasia methods.
- Do not list animals as materials.
- Indicate which institutional and national guidelines for the care and use of laboratory animals were followed.
- Confirm that the study went through a process of ethical review prior to the study commencing, indicate which ethics committee approved the study and provide the associated permit number(s).
- If ethical approval was not required, include a statement of this and the reason.
- Confirm that the potential for application of the 3Rs was rigorously researched prior to starting, and every opportunity was taken during the course of the study to implement each of them.
- Confirm that animal husbandry and care was in accordance with contemporary best practice and all individuals involved with the care and use of animals were trained and skilled to an acceptable level of competency, with euthanasia carried out according to contemporary best practice.
-
- JARO recommends following the PREPARE guidelines (https://norecopa.no/PREPARE) in planning studies using animals to ensure that the above requirements are met and the respective information is documented during the study. JARO further recommends the use of the Experimental Design Assistant (https://www.nc3rs.org.uk/experimental-design-assistant-eda) for the design of experiments using animals to ensure that they use the minimum number of animals consistent with their scientific objectives, methods to reduce subjective bias and appropriate statistical analysis.
- Key resources Research Resource Identifiers (RRIDs) should be provided for resources used in the study, such as novel genetic tools, transgenic animals, antibodies or other non-standard reagents. See the RIID Portal (https://scicrunch.org/resources) for more information about RIIDs.
- Equipment Manufacturer, city, state (if applicable) and country must be given.
- Chemical substances Chemical substances must be properly identified. Except for standard laboratory chemicals, the source of supply (supplier’s name and country) must be given. Drugs must be identified by the generic or official name wherever possible. Proprietary names should be avoided.
- Buffers and incubation media Compositions of incubation media should be described, or a reference supplied, together with the pH. Concentrations of solutions should be described in molar terms (mol/l and subunits thereof), equivalents, or percentage weight/volume (wt/vol.) or weight/weight (wt/wt). Mass concentration should be expressed as g/l (or subunits thereof—mg/l or µg/l). It should always be made clear whether concentrations in a mixture are final concentrations or those of solutions added.
- qPCR The journal supports the use of the Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines for the reporting of quantitative real-time PCR experiments. We also recommend the use of multiple normalization genes to be tested for their stability using geNorm (Vandesompele J, De Preter K, Pattyn F, et al (2002). Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3, RESEARCH0034. https://doi.org/10.1186/gb-2002-3-7-research0034).
- Genes and proteins Italic characters should be used for gene symbols including genotypes, alleles, mRNA, etc. Upright font and uppercase letters are generally used for protein abbreviations. For example:
-
- Human gene: XYZ
- Rat/mouse gene: Xyz
- Protein (any species): XYZ
For further details, please see the guidelines recommended by the Human Gene Nomenclature Committee, or by the Jackson Laboratory.
- For work that relies on antibodies, the methods section should include information about how antibodies were validated either by citing prior work, e.g., antibodies listed in the JCN database (https://onlinelibrary.wiley.com/page/journal/10969861/homepage/other_resources.htm) or (preferably) in the Antibody Registry (http://antibodyregistry.org). Alternatively, authors may provide a full blot image (not cropped) for each antibody to demonstrate protein specificity; if possible, they should include an evaluation of staining in a knock-out animal.
- For studies using analysis of images, the analysis section should also include a description of all steps taken while acquiring images, image processing steps prior to analysis and any additional steps taken in the preparation of images for figures.
- The description of data analysis in the methods section should include all processing steps taken to analyze the data (including, but not limited to: additional software filtering, how samples and/or subjects and technical replicates were pooled to determine sample sizes, whether aspects of the experiments were randomized or not, whether the experimenters [and subjects, if relevant] were blinded to subject treatment, and if blinding was used, at what point in the analysis such blinding was unmasked).
Results
Present only original, unpublished results. The same data should not be presented in figures and tables. Do not repeat all the data that are set out in the tables or figures in the text; emphasize or summarize only important observations. All figure sections should be mentioned in the text.
Discussion
The Discussion should deal with the interpretation of the results and not recapitulate them. We encourage authors to write their Discussion in a structured way, as follows:
- Statement of principal findings;
- Strengths and weaknesses of the study;
- Strengths and weaknesses in relation to other studies, discussing important differences in results;
- Meaning of the study, including possible explanations and implications for clinicians and policymakers;
- Unanswered questions and future research.
Acknowledgments
Make appropriate acknowledgements (to collaborators, technical support and sources of financial support) in a separate section after the Discussion. Please provide names (initials and surname) and affiliations.
Funding
Please include a separate Funding section after your Acknowledgements that details your sources of funding. Any grant support that requires acknowledgement should be mentioned. The names of funding organizations should be written in full. Where no specific funding was received, please insert the following statement: “This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.”
Role of study sponsor or funder
The ICMJE uniform requirements for manuscripts submitted to medical journals state that authors should describe the role of the study sponsor or funding source, if any, in the study design; collection, analysis, and interpretation of the data; writing the report; and any restrictions regarding the submission of the report for publication—for example, “The sponsor/funder provided editorial assistance only.” or “The sponsor/funder was involved in study design and data collection only.” If the sponsor/funder had no such involvement, the authors should state “The study sponsor/funder was not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.”
Authors’ relationships and activities
Authors are responsible for recognizing and disclosing relationships and activities that readers could perceive to have influenced, or that give the appearance of potentially influencing, their work. They should acknowledge in this section any industry links and other personal connections.
Data availability
Please include a statement of data availability. This should include information on where data supporting the results reported in the article can be found (including, where applicable, hyperlinks to publicly archived datasets analyzed or generated during the study), or whether the data are available on request from the authors or if no data are available. Springer Nature provides examples of data availability statements.
Contribution statement
The ICMJE uniform requirements for manuscripts submitted to medical journals state that authorship credit should be based on:
- Substantial contributions to conception and design, acquisition of data or analysis and interpretation of data;
- Drafting the article or revising it critically for important intellectual content;
- Final approval of the version to be published;
- Agreeing to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
All four conditions must be met by all authors. Participation solely in the acquisition of funding or data or general supervision of the research group does not constitute authorship.
Please include a statement listing each author’s contribution. Please ensure that this is discussed with your co-authors and agreement is reached prior to manuscript submission. Post-acceptance changes to the author list will not be permitted.
In accordance with the Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals (ICMJE Recommendations 2013), please identify the guarantor(s) at the end of the Contribution Statement. The guarantor accepts full responsibility for the work and/or the conduct of the study, had access to the data and controlled the decision to publish.
References
References to the literature should be credited to the original findings and be in numerical order in the text, the number being given in square brackets on the line, and numbered in the same order at the end of the manuscript. References should not be used by authors, editors or peer-reviewers to promote self-interests. There must be only one reference per number. Reference may only be made to Abstracts published in the current or preceding year. References to material on a preprint server should be included in the reference list using the format below, but should be replaced by the peer-reviewed, published version if this becomes available.
If you are using Endnote, right-click and save this link to download JARO's Endnote style:
If you are using Zotero, right-click and save this link to download JARO's Zotero style:
Reference list In accordance with Springer publishing policy, all references should be in the ELSE-Ciba style, as follows:
Articles in journals Names of up to six authors with initials (seven authors or more should be abbreviated to et al after the third author’s name); (year); title of paper in full; abbreviated name of journal (according to the NLM Catalog); volume number (issue number); first and last page numbers (full page range). DOI, e.g., Meltser I, Cederroth CR, Basinou V, Savelyev S, Lundkvist GS, Canlon B. (2014) TrkB-mediated protection against circadian sensitivity to noise trauma in the murine cochlea. Curr Biol 24(6):658-63. https://doi.org/10.1016/j.cub.2014.01.047. 99(19):12363–12368.
Whole book Names of all authors with initials; (year); title of book; edition; name and city of publisher, e.g., Møller AG, Langguth B, Ridder D, Kleinjung T (2011) Textbook of Tinnitus. Springer, New York, NY.
Chapter from a book Names of all authors with initials; (year); chapter title; In: editors’ names with initials (eds); title of book; volume number; name and city of publisher; pp first and last page numbers, e.g., Roberts LE (2011). Neural Synchrony and Neural Plasticity in Tinnitus. In: Møller, A.R., Langguth, B., De Ridder, D., Kleinjung, T. (eds) Textbook of Tinnitus. Springer, New York, NY. https://doi.org/10.1007/978-1-60761-145-5_13
Letters to the Editor As for articles in journals, e.g., Cederroth CR (2012) Loss of aminoglycoside sensitivity in HEI-OC1 cells? Hear Res. 292(1-2):83-5
Material on a preprint server This should include “(preprint)” and the version date, as well as the date you accessed the material, e.g., Genitsaridi E, Kypraios T, Edvall NK, et al. (2020). The spatial percept of tinnitus is associated with hearing asymmetry: subgroup comparisons. MedRxiv 20073999 (Preprint). 08 May 2020. Available from: https://doi.org/10.1101/2020.05.05.20073999 (access date)
Website Authors; (year); title; URL; date accessed, e.g., Regional Office for the Western Pacific of the World Health Organization, International Association for the Study of Obesity and the International Obesity Task Force (2000) The Asia-Pacific perspective: redefining obesity and its treatment. Available from www.obesityasiapacific.com. Accessed 10 October 2003
Papers quoted as “in press” Authors should provide an electronic version of any manuscripts cited as “in press” when they submit their manuscript to JARO. If an accepted paper contains references to a manuscript “in press,” written evidence that the manuscript has been accepted will be requested. Numbered references to personal communications, unpublished data and manuscripts either “in preparation” or “submitted for publication” are unacceptable. If essential, such references may be incorporated in parentheses in the appropriate place in the text, but written consent for publication must be provided.
The references are the responsibility of the authors. They must be written correctly and be rechecked by the authors in the proofs. References to abstracts (only current and preceding year), letters to the editor, congress proceedings and non-peer-reviewed publications should be kept to a minimum.
Equations
Simplify equations as much as possible and set in the manuscript exactly as they should appear in the paper. The use of LaTeXIt or MathType to insert the equations as images is acceptable.
Permissions
Lengthy direct quotations (100 words or more), illustrations or tables that have appeared in previously published material must be accompanied by written permission for their use from the copyright holder (normally the publisher).
Footnotes
Do not use footnotes, unless with exceptional need. Incorporate explanatory material into the text.
Units
Use the metric system of units and SI (Système International) format—e.g., s instead of sec.
Figure format
Labels and wording in figures
- If possible, please use editable vector fonts for all labeling and lettering in your figures.
- All figures should be numbered using Arabic numerals (Fig. 1, Fig. 2, etc.) and figure parts should be labeled with lower case letters (Fig. 1a, b, etc.).
- Use a sans serif font for numbers and lettering: Helvetica if available, otherwise Arial. Please use the same font for all figure labels in your manuscript.
- Courier font can be used for sequence alignments.
- The font size should be selected so that it is easily readable (final size 2–3 mm, 8–12 pt) when the figure is reduced for publication. This means that the font may need to be considerably larger when preparing the figure, so that it will be 8–12 pt once the image has been reduced.
- Please use a consistent font size for x- and y-axis labels and across different figure parts.
- Labels should be in sentence case (the first letter of the first word uppercase and the rest of the label lowercase, except for proper names, abbreviations, etc.).
- Numbers on the x- and y-axes should read horizontally wherever possible.
- Symbols on line graphs should be clear and easy to distinguish from one another. If there are only two symbols used, it is clearest to use two different color fills, e.g., one black symbol and one white. Otherwise use, for example, black squares, white squares, black triangles, white triangles, etc. For scatter diagrams and line graphs, solid symbols are preferred.
- If the axis shows a measurement, please ensure that units are included in parentheses at the end of the axis label, e.g., “Plasma glucose (mmol/l).”
- Please use SI units for all measurements in figures and in the main text. For an SI conversion table, please see the AMA Manual of Style conversion calculator.
- Please ensure that the terminology, formatting and spelling in figure labels is the same as in the text. For example, where gene symbols are included, please use gene formatting (italic), and please also use U.S. English spelling.
Multipart figures
- Each figure part should have its own part label (a, b, c, etc.), with the following exceptions, which can be treated as one part: gel or blot with associated quantification graphs; line graph with inset AUC graph; composites of micrographs (including associated quantification graphs).
- Figure parts should not be supplied as individual files; please incorporate them into a composite figure as you would like them to appear in the final version of the paper. When deciding on the layout, please consider how it will look in print, and how clear it will be to readers. Try to minimize white space.
- The composite figure should be sized to an appropriate size for typesetting. Generally, figures will be set in a single column (8.4 cm) or less with the legend below, or in 1.5 columns (12.9 cm) with the legend to one side. These sizes may be changed slightly by the Editorial Office to ensure consistency. Larger composite figures will be set at up to full page width (17.4 cm). The maximum page height is 23.5 cm.
- Figure layout will sometimes be changed by the Editorial Office to make sure that space is optimized.
- Separate parts should be sized so that they are consistent across the figure; similar graphs should be the same size, and font sizes should be the same throughout the figure, as far as possible.
- Separate figure parts should be labeled with lowercase letters and should be in alphabetical order (either horizontally or vertically).
- Each individual graph should have its own x- and y-axis labels, even where these are repeated along rows or columns of graphs.
- If possible, use editable vector fonts for all labels and symbols, by using a vector program such as Adobe Illustrator or PowerPoint to put together your figures. See the above “Labels and wording in figures” section for more information.
- Halftone images can be prepared in Photoshop and then inserted into Adobe Illustrator or PowerPoint for layout and labeling.
- Where images are inserted into other programs, such as PowerPoint, Adobe Illustrator or Photoshop, please ensure the image is inserted at the correct resolution. This can generally be done by using the ‘insert’ function, provided the image file to be inserted is at the right resolution. Cutting and pasting will result in a low-resolution image.
Graphs and diagrams
- To keep figures uncluttered, please omit headings, captions, keys and statistical values; this information should be included in the main figure legend instead.
- Please use standard statistical notation in figures (*p < 0.05, **p < 0.01, ***p < 0.001). The usual order for symbols for different comparisons is: *, †, ‡, §; please do not use # or $ signs.
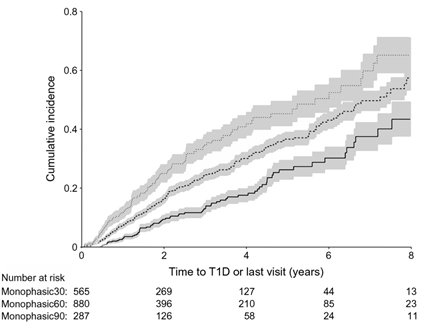
- Please provide exact n values for numbers of samples for each figure part, rather than, for example, n = 3–6. For complex experiments including a variable number of samples and replicates, consider including this information in a table under the x-axis (see example below).
- Please avoid borders or frames around graphs. Do not use gridlines.
- Please use a weight of approximately 0.75 pt (3/4 pt) for axis lines and lines within graphs. Axis lines should be black.
- Where dashed lines are used, dashes should be sufficiently large and spaced to be seen clearly when figures are reduced.
- Curves should not be drawn beyond experimental points.
- Data should not extend beyond the upper or lower values shown on the axis.
Bar charts
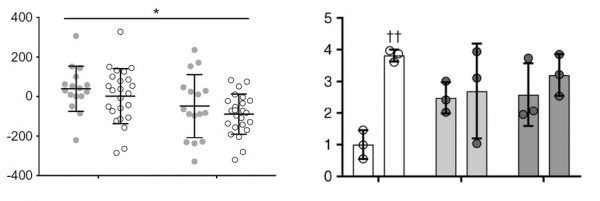
- Present data as individual points rather than as simple bar charts. This can either be in the form of a bar chart with individual data points shown clearly on the graph, or a column scatter graph, as shown in the examples below.
- Different symbols can be used for different samples or treatments, if appropriate.
- Please use lines to show the means and error bars, and make sure these can be read clearly.
- If bar charts are required (e.g., to avoid crowding where there are a large number of samples), fills should be easy to distinguish. If you need to use a patterned fill, widely spaced horizontal or diagonal stripes are most effective.
Forest plots
- If your forest plot presents data from multiple previous studies, please include the name of the study or the first author’s name followed by et al (give the names of both authors if there are only two); year of publication (optional); and the reference citation number [in square brackets]. Click here for an example.
- If possible, please provide forest plots as PowerPoint files in which the text is editable, or as EPS, PDF or Adobe Illustrator vector files. If this is not possible, please supply them as TIFF files at a resolution of 1200 dpi.
Flow charts
- Flow charts should be supplied as editable Word or PowerPoint documents.
- Please avoid use of bold font and use a weight of 0.5–1.5 pt for lines, borders and arrows.
- Please check that values add up where appropriate.
Schematic diagrams
- Schematic diagrams should be provided as editable PowerPoint files if possible. If other file types are supplied, authors may be asked to make stylistic changes themselves.
- Different styles can be used for boxes and shapes but, similarly to flow charts, please avoid bold and use a weight of 0.5–1.5 pt for lines, borders and arrows.
Halftones
- Halftones include color or grayscale graphs, images of gels, micrographs or photographs. Labels must be large enough to be read easily after reduction for publication and should contrast clearly against the background.
- If possible, please insert labels using a vector package such as Adobe Illustrator or PowerPoint. For example, images could be checked and cropped in Photoshop, and then imported into Adobe Illustrator or PowerPoint for any text and symbols to be added.
- The scale should be indicated in micrographs using an internal reference marker (scale bar) on the photograph itself (as a horizontal line at least 1 mm thick after reduction). The scale should be given in the legend (e.g., “scale bars, 20 nm”), rather than on the image itself.
- If photographs of patients/individuals are used, the individual should not be identifiable or the picture must be accompanied by written permission to use the figure.
Color images
- There is no charge for use of color in figures, and indeed the use of color is encouraged in figures.
- Please see the “Graphs and diagrams” section above for more information.
Embedding fonts
- For figures generated in Adobe Illustrator or Microsoft Office, or for other vector files, please embed the fonts when you save the document. This ensures that any non-standard fonts used will remain part of the file.
- Adobe Illustrator If you save in the Adobe Illustrator (AI) format, the subset of the font used in the image will be embedded automatically. If you save in the EPS format, please tick the “Embed fonts (for other applications)” box when saving.
- MS Office For Office 2013 and earlier: when you click “Save as,” the tools menu (next to the “Save” option in the dialog box) gives you the option of embedding fonts. For Office 2016 onward: first, click on the File tab and then select “Options.” In the dialog box, select “Save” and check the “Embed fonts in the file” box at the bottom of the page.
Figure format
If your paper is accepted, you will be asked to supply original vector files if possible, or high-resolution TIFF files. Graphs and diagrams are best provided as EPS vector files, or in another vector format such as PowerPoint, Excel or PDF, so that lines and text will remain clear when the figure is resized for publication. Vector-based images are made up of lines rather than pixels. Because they are not composed of a specific number of dots, they can be scaled to a larger size without loss of image quality. Resolution, which is important for non-vector files, is not an issue for vector files because of this scalability. Supplying vector files in one of the formats shown below will ensure that the final print quality of the images is good.
Because of file size limitations, you may wish to upload lower resolution versions onto ScholarOne Manuscripts for review purposes when submitting your paper. Higher resolution figures will be requested if your paper undergoes revision or is accepted.
The following file types are preferred:
Vector
- EPS
- AI
- PowerPoint
- Excel
- Word (generally acceptable only for flow charts/schematics)
Non-vector
- TIFF
- PNG
Notes on particular software packages
- Stata and GraphPad For information on converting figures created in Stata and GraphPad to EPS vector files, please see our detailed instructions.
- SPSS Graphs created in SPSS cannot always be saved with sufficient resolution for publication, although we have been advised by SPSS/IBM that exporting as a PDF is likely to give the best results. Depending on the clearness of your images, you may need to replot data from this program in an alternative program.
- Microsoft Office The resolution of TIFF files saved from Microsoft Office is not usually high enough for publication, so please send the original Microsoft files (PowerPoint, Excel, Word).
TIFF files
- Resolution is important for these non-vector files because the image will be fuzzy and pixelated if the resolution is not high enough.
- For non-vector files, please supply TIFFs saved at a resolution of 1200 dpi for black and white graphs, and 600 dpi for graphs containing grey or colored fills, gels, blots, micrographs and composite images (a mixture of line graphs and other images).
- When increasing the resolution of figures, please avoid resampling (e.g., in the “Image size” function in Adobe Photoshop), as resampling will result in the artificial addition of pixels (essentially adding data by guesswork), which may lead to inaccurate or blurry images.
- TIFF files created in Photoshop or similar artwork software should not be flattened.
- When saving graphs and line art as TIFF files, select “none” for compression, if possible; for halftones use LZW compression.
- Color images should be encoded as RGB (8 bits per channel) rather than CMYK. For graphs and flow diagrams, please select colors from the JARO color palette. The extended color palette may be used for schematics and diagrams only.
Tables
- Include tables in the submitted manuscript after the figure titles and legends. Tables should not be saved as figures (i.e., as .jpg or .tif files). All tables intended for print should be incorporated at the end of the manuscript Word file. Tables should not be uploaded individually.
- When creating a table, please use the Microsoft Word table function, and please do not place an Excel table into a Word document. Tables not created with the Microsoft Word table function will be sent back for revision. Do not submit a table in PDF format.
- Word tables should not be tab or space delineated and should not include colored text or shading, but embedded graphics with color are okay.
- Do not use paragraph returns to separate data within a cell.
- Tables should include a title, and footnotes and/or legends should be concise.
- Table titles may not contain parenthetical information, reference citations or footnote citations.
- Use superscript lowercase letters (beginning with “a”) for footnotes in tables. Do not use numbers or symbols.
- Tables must be numbered as Table 1, Table 2, Table 3, etc., rather than as Table 1a, Table 1b, Table 1c, etc.
- If italic font is used within a table to indicate some feature of the data, an explanation of its meaning must be given in the table legend. Bold text may not be used in tables.
- If a referenced paper or study is mentioned within a table, it must be included in the references list and must be followed by its appropriate citation number (e.g., “Author et al.1”) within the table.
- All abbreviations within a table must be defined in the table legend or footnotes.
JARO strongly recommends that authors obtain the advice of a statistician at their institution or elsewhere prior to starting a study, to aid in the study design. If they seek such advice, the resource should be identified in the letter of submission, and in the acknowledgements. Any statistical analysis must be checked for accuracy by the authors; if statistical software is used, the source (including version number) of the tools must be listed in the Materials and Methods; this information should be provided in the final paragraph.
Statistics should be fully reported in the manuscript/article. This includes stating the statistical test(s) used, the exact value of N (sample size) and the definitions of the distribution center (mean, median) and measures of variability (SD, SEM and confidence intervals) reported in the text and shown in figures. Note that many tests have multiple variants, so saying “t-test” is insufficient. For example, for t-tests, please specify paired or unpaired Student's or Welch's and single- versus two-sided (which will critically depend on the hypothesis being tested). If an unusual test is used, please justify it.
All legends should include specific “N” for each treatment group and a description (or brief description) of statistics used for each experiment. The definition of a sample should be made clear (N cells, M subjects). If data are pooled from multiple observations on individual subjects, the operations involved in computing the pooled value must be stated.
All statements that indicate the presence of “significant effects” should be supported by an appropriate statistical test. State the type of test that was used, including the degrees of freedom, the resulting test value and the exact p-value (to two significant figures) that the result occurred at chance under the null hypothesis.
Format: The format of the description of the statistical results should indicate the degrees of freedom, the statistic value, and the p value, as in these examples:
|
F(3,21) = 5.62, p = 0.0054 |
|
t(7) = 4.582, p = 0.0025 |
|
r2(9) = 0.77, p = 0.0004 |
To avoid ambiguities, all statistical variables should be italicized (F, t, r, p).
The type of post hoc tests used when following any analysis of variance (ANOVA) with multiple comparisons should be identified.
Reporting p values with inequalities should be limited to data grouped in tables or figures, or for post hoc tests (multiple comparisons) if no exact value is reported by the software.
Manuscripts that report results based on the analysis of large datasets, including (but not limited to) genomic sequencing studies and functional magnetic resonance imaging studies, are also required to specify in detail how the statistical analyses were done.
Although we do not recommend this as a regular practice, if the statistical analysis has been extensive, the results may be placed in a table, and if the table would be quite large and not suitable for a printed page, we will consider the authors’ request to place the table in a Supplemental material section. Such tables must provide all of the same information requested above, and must be appropriately cross-referenced to the text.
The contents of supplemental files are restricted to (1) figures that cannot be rendered in print with enough detail to be informative; (2) tables that have too many columns and/or rows to fit across two printed pages; (3) clinical descriptions, tables and figures that would substantially lengthen the print version of the manuscript; (4) movies; and (5) supplemental methods (see below).
For full-length articles, supplemental material and methods should be limited to those materials or methods related to the supplemental display items, detailed analytical methods and tabular presentations of primers or other information that would not fit well in the main text for formatting reasons.
We encourage authors to provide complete descriptions rather than referring to previous publications. Please note that the main paper also needs to give brief explanations of all the methods with enough detail to allow readers to understand the general experimental design and the results of the experiments. Any tables, such as a list of primers, included in the supplemental material and methods should not be numbered.
Supplemental information should be provided with the original submission. Please follow the figure guidelines below for preparing figures. All figures and tables should have titles and legends.
Please provide a single PDF that contains all supplemental case reports, figures and legends; supplemental tables; and supplemental references (in this order). If a supplemental table cannot fit onto two 8.5” × 11” pages, please instead supply the table as an Excel file. Please do NOT include the title or author list in the PDF; we will add a coversheet with this information. Please also do not include movie titles and legends; leave those in the main text, and we will move them to a separate online page that links to the movies. In addition, please do not include page numbers in your final PDF. We strongly recommend that the final size of this PDF be less than 10 MB in order to ensure successful downloads for all readers. In addition, this PDF should be considered the FINAL version; it will be published as is, except for the coversheet that we will add. Scientific errors detected in the supplemental information after publication will require that a correction be published (as with errors in the main paper).
Please follow the following style preferences to ensure that your supplemental PDF is consistent with the copyedited version of your main text:
- Case reports should be titled “Supplemental note: case reports.” These descriptions should fully describe the phenotypes of the affected individuals and are not subject to a word limit.
- Figures should be titled Fig. S1, Fig. S2, etc. (NOT Supplemental Figure 1, Supplemental Figure 2, etc.)
- Similarly, tables should be titled Table S1, Table S2, etc. (NOT Supplemental Table 1, Supplemental Table 2, etc.)
- Please use the word “Supplemental” rather than “Supplementary” in headings (e.g., “Supplemental Material and Methods,” “Supplemental References,” etc.)
- As with main-text figures, supplemental figures should include error bars where appropriate, and these error bars should be clearly defined in the figure legends
Videos
Video illustrations for online-only material (Supplemental videos): Suitable formats are: MPEG (.mpg; preferred), .rm, .avi and .mov.
If you are asked to revise your manuscript, you will be expected to provide a covering letter that responds in detail to each point raised by reviewers or editors, and to indicate, using a different color font, all changes and new material in your paper, ensuring that such changes will be clear if referees print your manuscript in black and white (do not use the “Track changes” mode in Word).
Upon acceptance of your article, you will receive a link to the special Author Query Application at Springer’s web page where you can sign the Copyright Transfer Statement online and indicate whether you wish to order Open Choice or offprints. Once the Author Query Application has been completed, your article will be processed and you will receive the proofs.
Copyright transfer
Authors will be asked to transfer copyright of the article to the ARO (except in Open Choice articles). This will ensure the widest possible protection and dissemination of information under copyright laws. If you transfer copyright to the ARO, then your article will be available to all ARO members and institutional subscribers to JARO for the first year, but not to the public at large until a year has passed. As soon as your article is published online (in “Online First”), Springer will automatically upload your article to PubMed Central with a one-year embargo. This procedure will satisfy the requirements for research funded by the U.S. National Institutes of Health.
Open Choice
In addition to copyright transfer, Springer provides an alternative publishing option which you may choose in the pre-publishing period: Springer Open Choice. If you do not wish to transfer copyright and/or wish to make your paper freely available before one year after the publication date, you can do so by choosing Springer Open Choice for $3,000 at the time your article is accepted for publication. In that case the article will also be available immediately in PubMed Central, as well as on SpringerLink.com. (Springer Open Choice cannot be ordered for already-published articles.) Open Choice articles do not require transfer of copyright as the copyright remains with the author. In opting for open access, authors agree to the Springer Open Choice License.
Proofs
The purpose of the proof is to check for typesetting or conversion errors and the completeness and accuracy of the text, tables and figures. Substantial changes in content such as new results, corrected values, title and authorship are not allowed without the approval of the Editor. After online publication, further changes can only be made in the form of an Erratum, which will be hyperlinked to the article.
Online First
The article will be published online after receipt of the corrected proofs. This is the official first publication citable with the DOI. After release of the printed version, the paper can also be cited by issue and page numbers.
Manuscripts are submitted and reviewed via the online manuscript submission and review system, Editorial Manager. For the initial submission, you may submit in the formats listed below OR a PDF, which may or may not include all figures. If you do initially submit a PDF, note that you will have to submit a non-PDF format (below) with individual submission of figures before the manuscript can be accepted. Submission of the text version of the manuscript (usually in Word doc/docx or LaTeX format) should occur with the first request for revision. For final submission, acceptable formats for the text are Word, RTF, LaTeX2E and TeX. The best formats for figures are TIFF (images, at 300–600 DPI) and EPS for line drawings (note that EPS files can also include images). Compressed formats such as JPEG or GIF are not recommended.
Format manuscripts for standard letter (8-1/2” × 11”) or A4 paper with single spacing throughout, including the Abstract, text, acknowledgments, references and figure captions, and wide (> 1” or 2.5 cm) margins. Continuous line numbers should be included in the left margin. Number all pages, beginning with the abstract page, at the bottom.
Manuscripts that do not meet these standards may be returned to the authors prior to review for corrections.
JARO has joined the NPRC, an alliance of neuroscience journals that have agreed to share manuscript reviews at the author’s request. The NPRC has been formed to expedite the review process, to speed the publication of research reports and to reduce the overall burden on peer reviewers. NPRC provides handshaking between journals that allow a formal transfer of reviews from one journal to another. For example, if a journal deems a manuscript to be too “specialized” for its readership, but otherwise has few faults with the work, the authors may choose to submit the manuscript to JARO (or another journal), along with the reviews. Thus, the manuscript has essentially been through the first round of review, and processing should be somewhat faster at the new journal. As a reviewer, you may see requests to review such manuscripts, and you may have reviewed them for another journal. With permission, reviewer names are forwarded, and we try to engage those reviewers with the resubmission to streamline the review process.
Likewise, if JARO does not accept the manuscript for publication, the authors may ask for the reviews to be forwarded to another journal in the consortium to expedite reviewing. Please let us know if you do not wish to allow your review to be forwarded in that case.
Reviewer names are never forwarded to authors, by JARO or any other journal in the NPRC. We strongly recommend, however, that you allow your name to go forward to editors of other journals, or it will be of little value. Please let us know when you accept the review if you prefer to not have your name forwarded to editors along with your review.